Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com
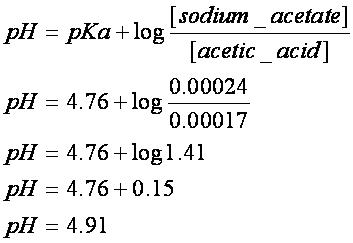
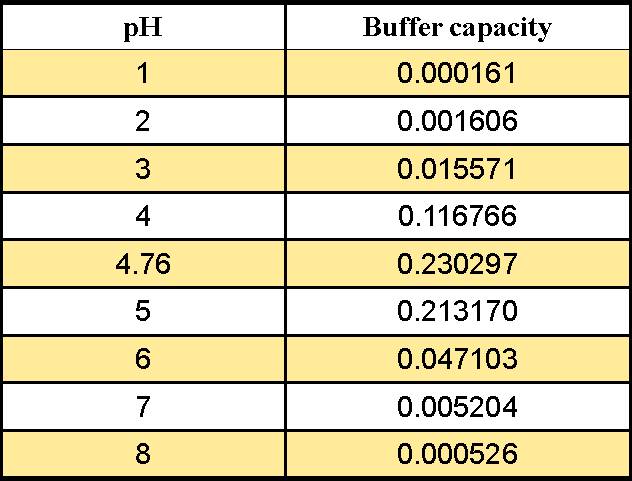
pH calculations and more in fundamentals of pharmaceutics. : Calculate pH of 100 ml buffer solution containing 0.1 g acetic acid and 0.2 g sodium actetate.

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com
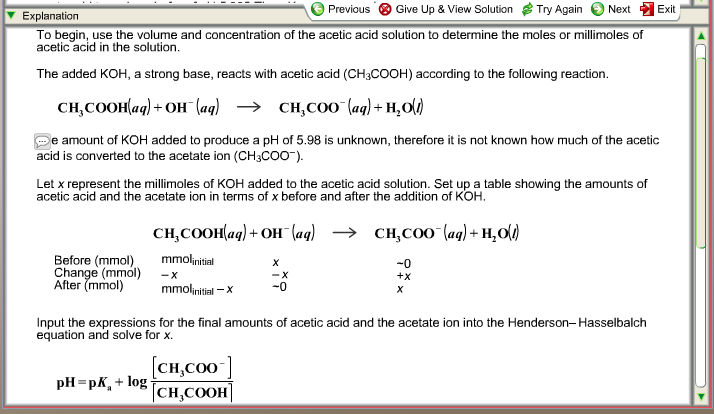
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://i.ytimg.com/vi/t9B5VgPOTG4/maxresdefault.jpg)
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]
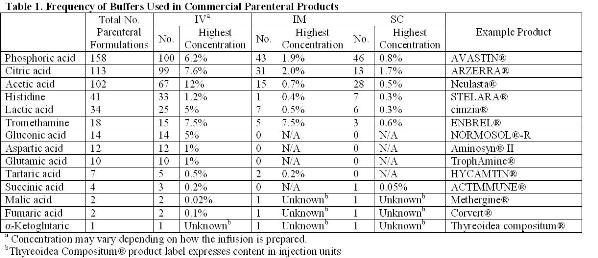
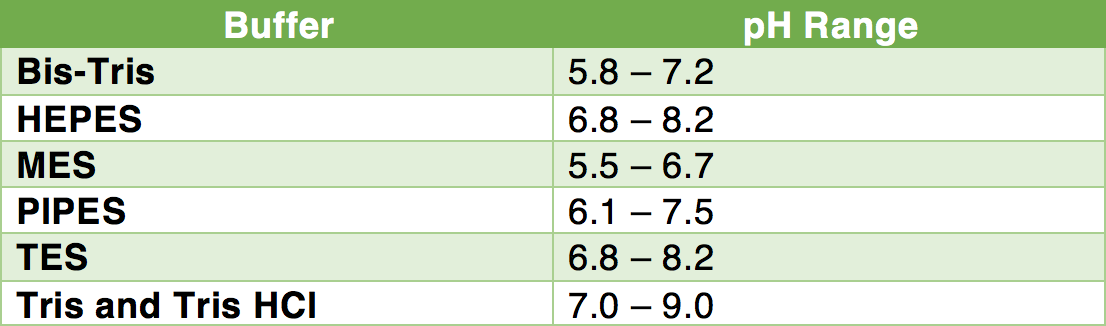
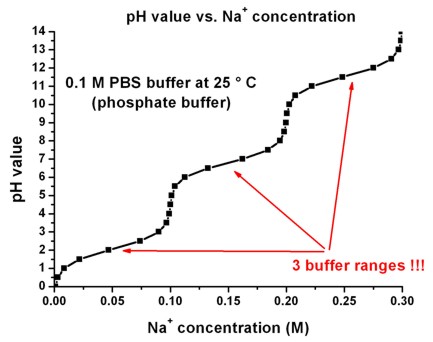
Breaking old habits: Moving away from commonly used buffers in pharmaceuticals - European Pharmaceutical Review
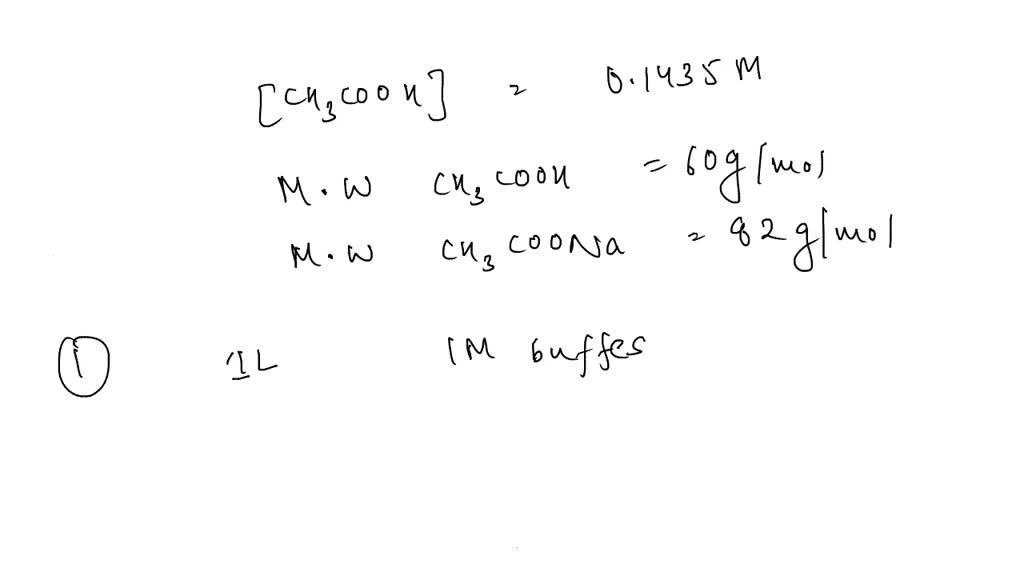
![Sodium Acetate [CH3COONa] Molecular Weight Calculation - Laboratory Notes Sodium Acetate [CH3COONa] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/sodium-acetate-molecular-weight-calculation.jpg)

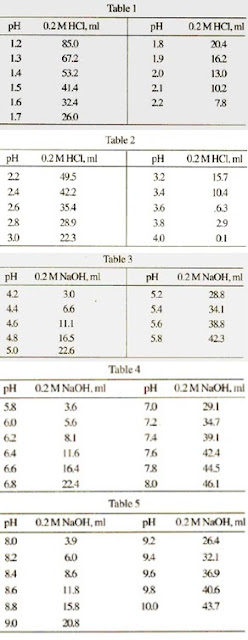

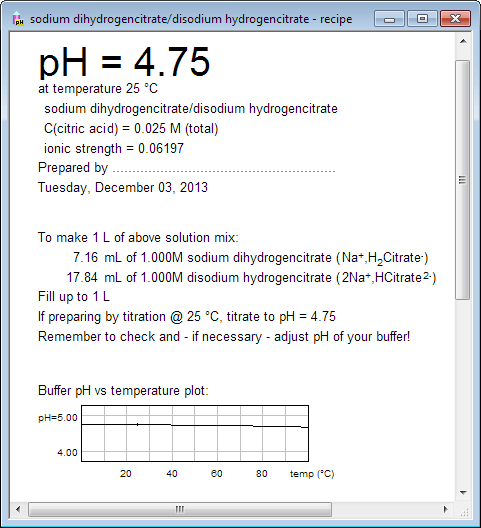

![BS093] 1.5M Sodium Acetate, pH 6.5 | Biosolution BS093] 1.5M Sodium Acetate, pH 6.5 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2016/02/BS005-Sodium-Acetate-Solution.jpg)