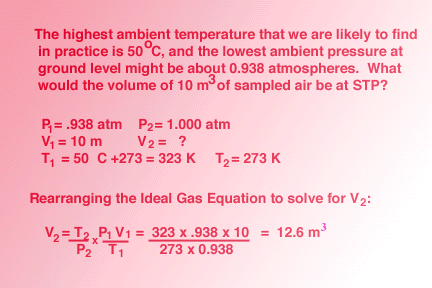
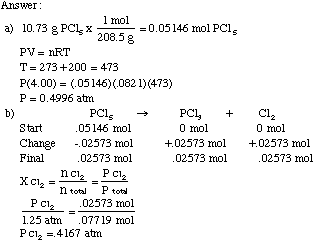
Five moles of ideal gas expand isothermally and reversibly from pressure 10 ATM to 2 ATM at 300 K. What is the largest mass which can be lifted through a height of

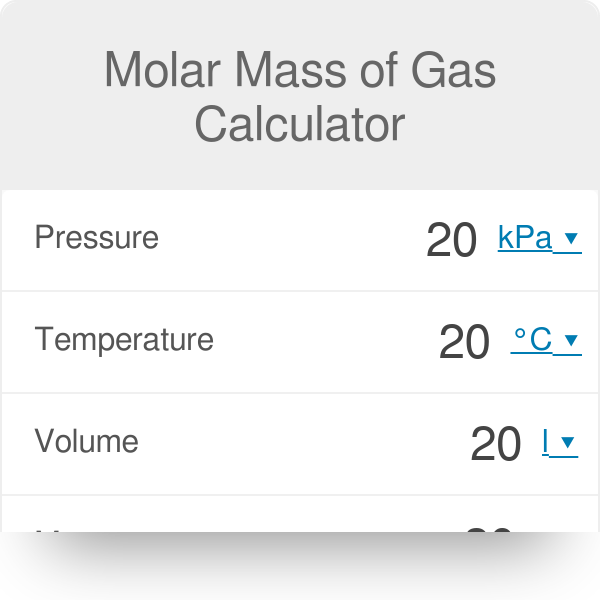
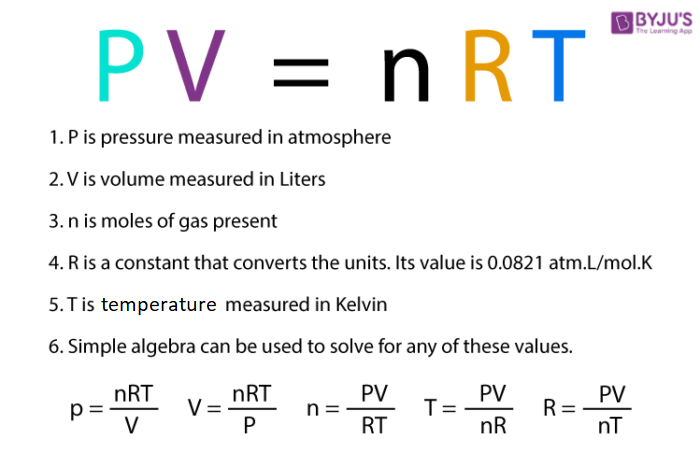
Ideal Gas Law Calculator (Pressure–Volume–Temperature–Amount) • Thermodynamics — Heat • Online Unit Converters

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

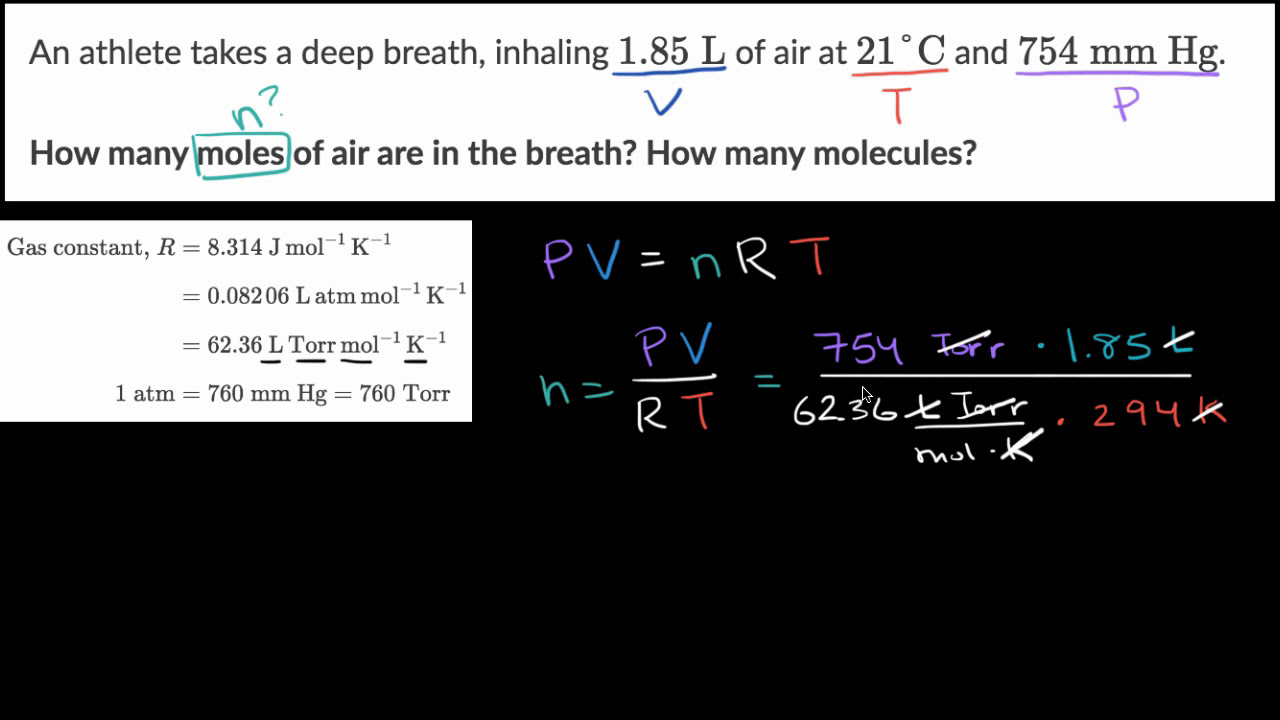
An ideal gas at 0.80 atmospheres and 87°C occupies 0.450 liter. How many moles are in the sample? - YouTube
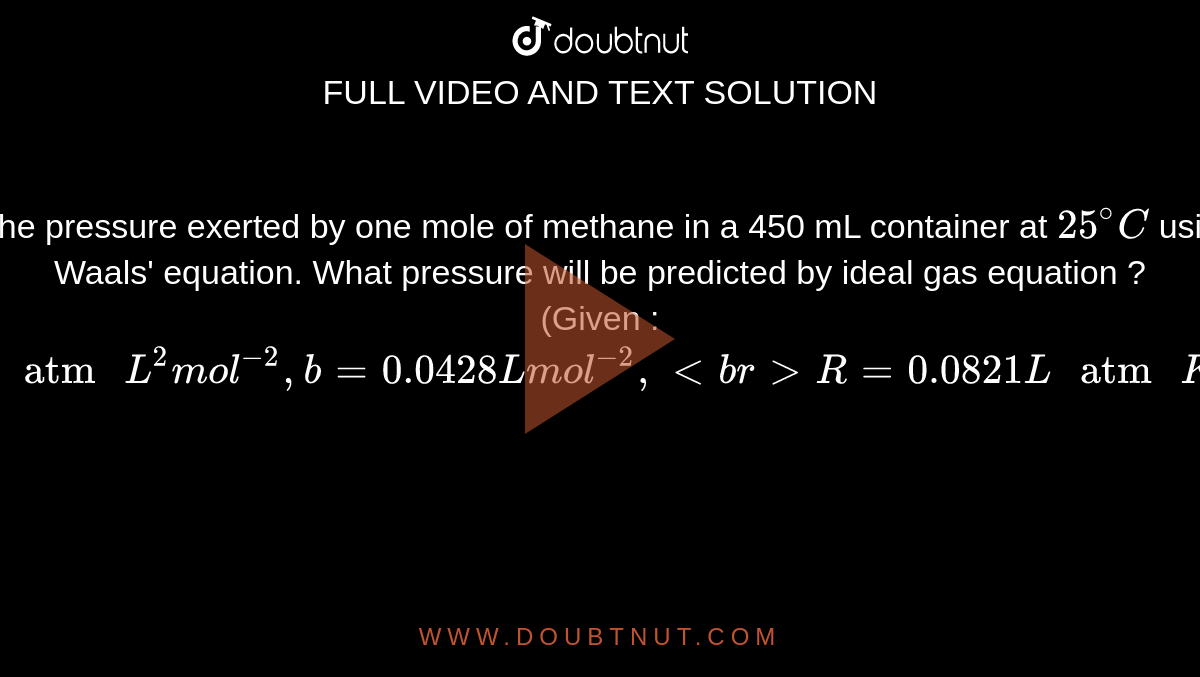
Calculate the pressure exerted by one mole of methane in a 450 mL container at 25^@C using van der Waals' equation. What pressure will be predicted by ideal gas equation ? (Given :

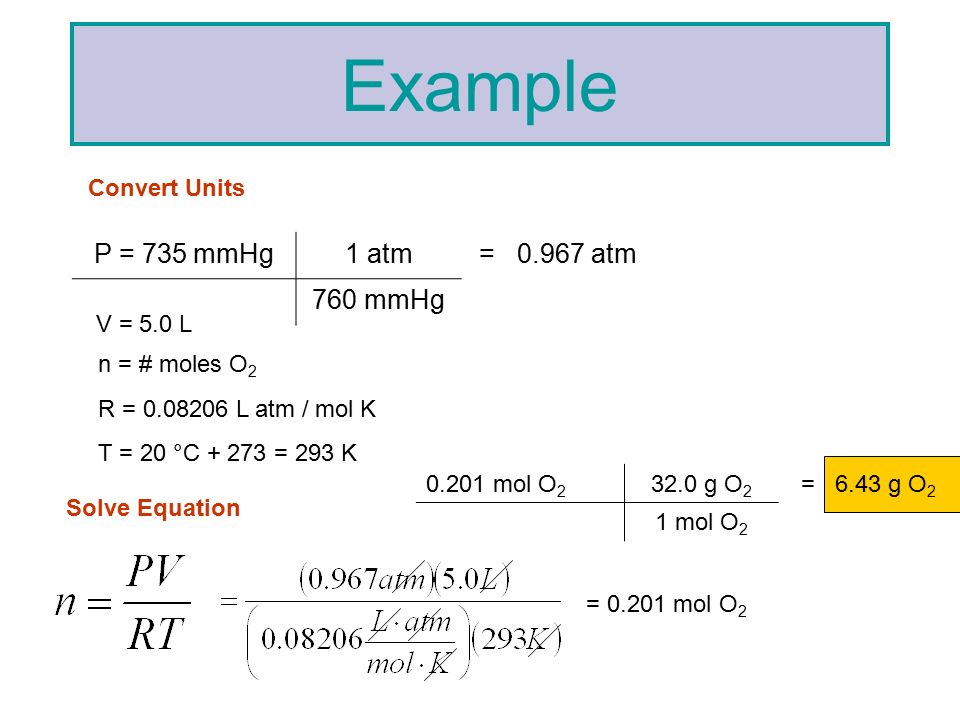
Calculate the temperature of 4.0 moles of a gas occupying 5 dm^3 at 3.32 bar (R = 0.083 bar dm^3 K^-1 mol^-1) .

Question Video: Calculating 𝐾_𝑝 at Equilibrium for a Mixture of Nitrogen, Hydrogen, and Ammonia | Nagwa