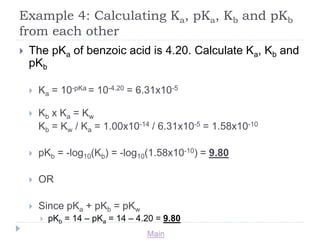
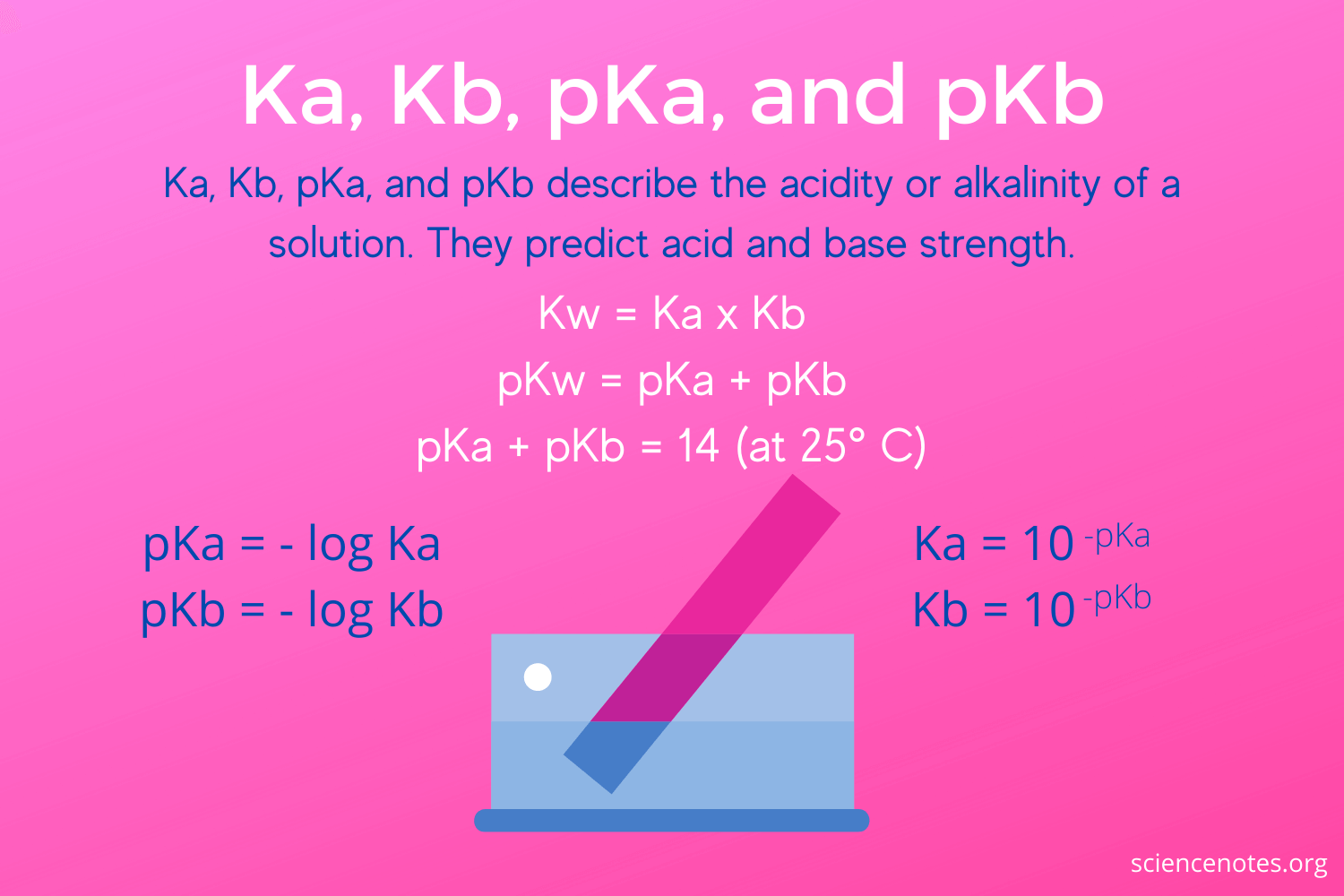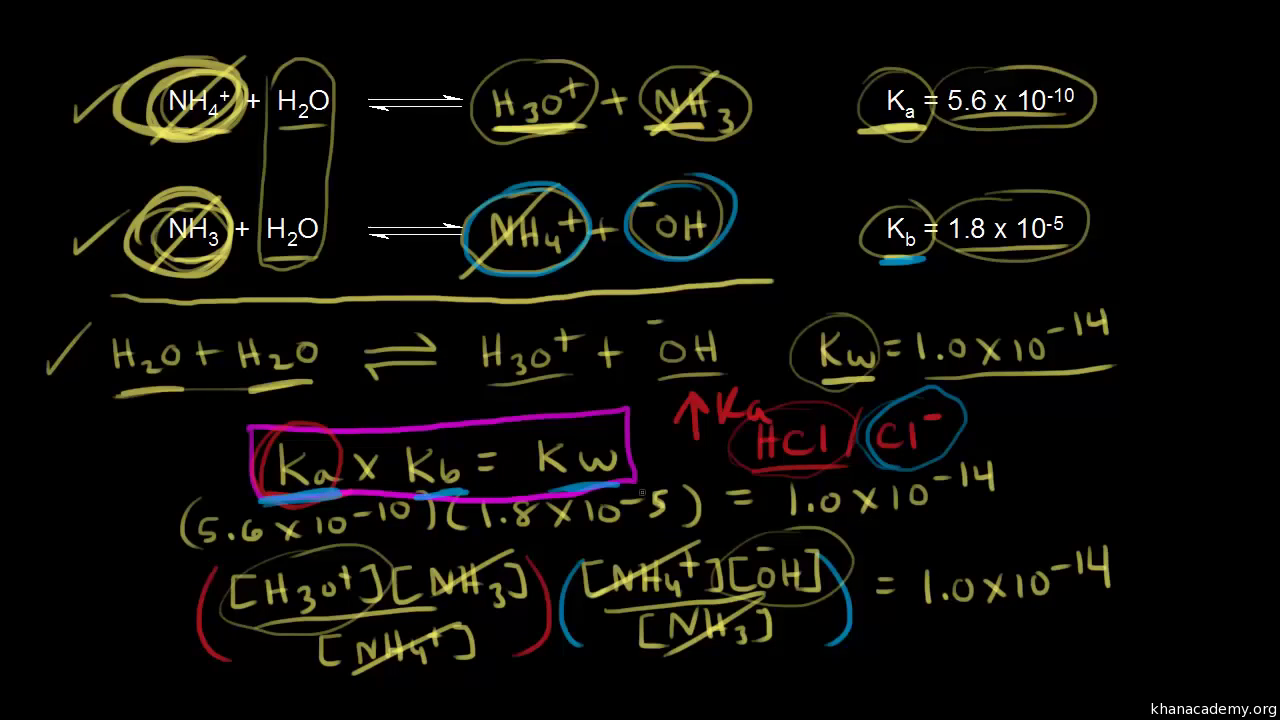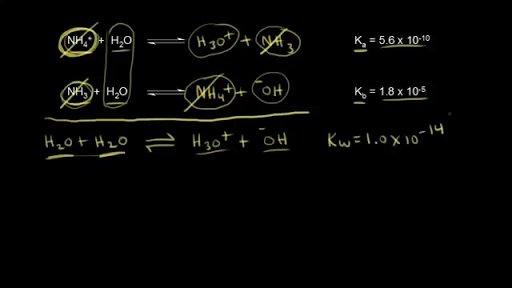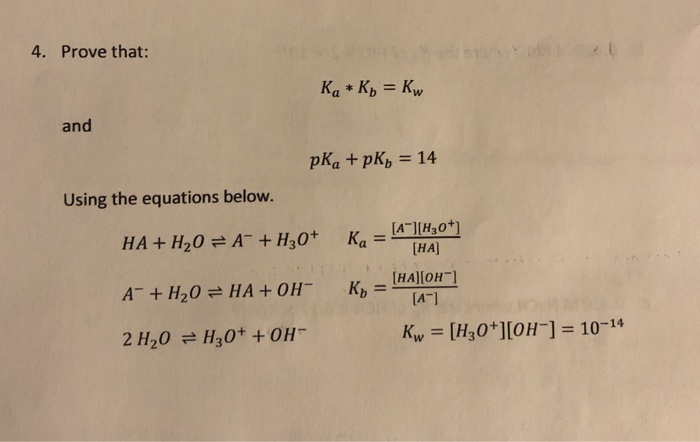SOLVED: How would you calculate Kb for the formate ion, given that the Ka for formic acid is 1.8 x 10-42 Kb= Ka * Kw d. Kb= Kw + Ka b Kb=

Acid-Base Equilibrium | Calculating the Ka or Kb of a Solution - Video & Lesson Transcript | Study.com

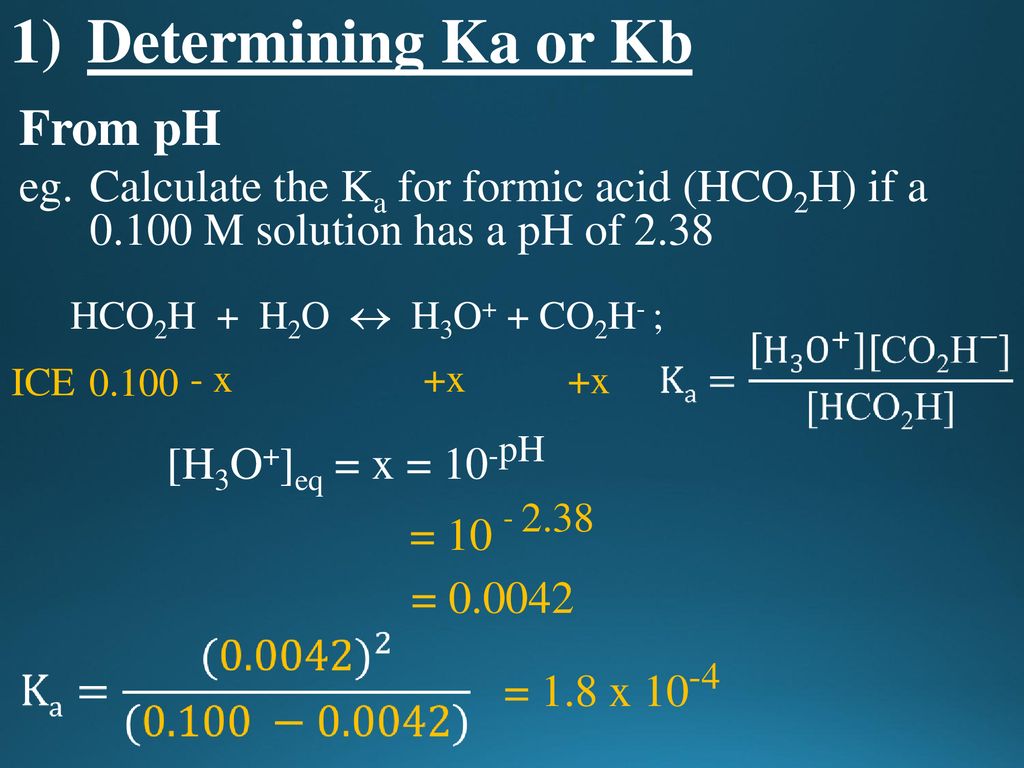
Calculate the pH of the following mixture given Ka = 1.8 × 10^-5 and Kb = 1.8 × 10^-5 ( pKa = pKa = 4.7447 ) 50mL 0.05M NaOH + 50mL of 0.1M CH3COOH

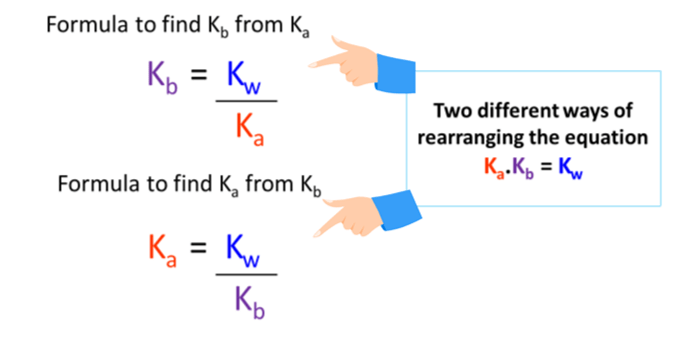
SOLVED: Question 7 (1 point) Saved How would you calculate Ka tor ammonium (NHa t) if you were given the Kb value tor ammonia (NH3)? Ka = Kbl Kw Oka = Kw/